Things have been really insanely busy on the work front over the last year, necessitating some radical prioritisation and thus I have only posted on Pharma Strategy Blog instead of here and Oncology Market Trends.
It will be changing from this week as things gear up for some interesting new perspectives on the Pharma CI front and I'd like to take the time to thank everyone for their patience during the hiatus.
Normal service will resume shortly.
Monday, August 24, 2009
Monday, October 6, 2008
2008 Nobel Prize for medicine honours 3 vaccine researchers
Somewhat controversially, the Nobel committee has awarded the Prize for Physiology and Medicine to three Europeans. Harald zur Hausen discovered human papilloma viruses (HPV), which causes cervical cancer and two Frenchmen, Françoise Barré-Sinoussi and Luc Montagnier made the groundbreaking discovery of human immunodeficiency virus (HIV) in 1983, a year ahead of the American scientist, Robert Gallo.
Zur Hausen discovered two high-risk types of the HPV virus and made them available to the scientific community, ultimately leading to the development of vaccines protecting against infection. Two vaccines, Gardasil and Cervarix, are now commercially available.
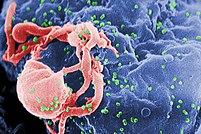 Image via WikipediaThe Nobel Assembly noted that Barre-Sinoussi and Montagnier's discovery was one prerequisite for understanding the biology of AIDS and its treatment with antiviral drugs. The pair's work in the early 1980s made it possible to study the virus closely. It allowed scientists to identify important details in how HIV replicates and how it interacts with the cells it infects. It also led to ways to diagnose infected people and to screen blood for HIV, which has limited spread of the epidemic, and helped scientists develop anti-HIV drugs.
Image via WikipediaThe Nobel Assembly noted that Barre-Sinoussi and Montagnier's discovery was one prerequisite for understanding the biology of AIDS and its treatment with antiviral drugs. The pair's work in the early 1980s made it possible to study the virus closely. It allowed scientists to identify important details in how HIV replicates and how it interacts with the cells it infects. It also led to ways to diagnose infected people and to screen blood for HIV, which has limited spread of the epidemic, and helped scientists develop anti-HIV drugs.
The French pair were embroiled in a heated debate throughout the 1980's with Dr. Robert Gallo. Gallo's dispute with Montagnier reached such a level in 1987 that the heads of State, Reagan and Chirac signed an agreement dividing millions of dollars in royalties from the AIDS blood test based on the two researchers' findings.
In the 1990s, however, the U.S. government acknowledged that the French deserved a greater share of the royalties. The admission solidified the French position that Montagnier had isolated the virus in 1983, a year before Gallo.
The press release for the announcement of the Medicine Prize is here.
Zur Hausen discovered two high-risk types of the HPV virus and made them available to the scientific community, ultimately leading to the development of vaccines protecting against infection. Two vaccines, Gardasil and Cervarix, are now commercially available.
 Image via WikipediaThe Nobel Assembly noted that Barre-Sinoussi and Montagnier's discovery was one prerequisite for understanding the biology of AIDS and its treatment with antiviral drugs. The pair's work in the early 1980s made it possible to study the virus closely. It allowed scientists to identify important details in how HIV replicates and how it interacts with the cells it infects. It also led to ways to diagnose infected people and to screen blood for HIV, which has limited spread of the epidemic, and helped scientists develop anti-HIV drugs.
Image via WikipediaThe Nobel Assembly noted that Barre-Sinoussi and Montagnier's discovery was one prerequisite for understanding the biology of AIDS and its treatment with antiviral drugs. The pair's work in the early 1980s made it possible to study the virus closely. It allowed scientists to identify important details in how HIV replicates and how it interacts with the cells it infects. It also led to ways to diagnose infected people and to screen blood for HIV, which has limited spread of the epidemic, and helped scientists develop anti-HIV drugs. The French pair were embroiled in a heated debate throughout the 1980's with Dr. Robert Gallo. Gallo's dispute with Montagnier reached such a level in 1987 that the heads of State, Reagan and Chirac signed an agreement dividing millions of dollars in royalties from the AIDS blood test based on the two researchers' findings.
In the 1990s, however, the U.S. government acknowledged that the French deserved a greater share of the royalties. The admission solidified the French position that Montagnier had isolated the virus in 1983, a year before Gallo.
The press release for the announcement of the Medicine Prize is here.
Monday, September 22, 2008
Does acupuncture help with breast cancer?
Acupuncture is as effective and longer lasting in managing the common debilitating side effects of hot flashes, night sweats, and excessive sweating (vasomotor symptoms) associated with breast cancer treatment and has no treatment side effects compared to conventional drug therapy, according to a study presented at the American Society for Therapeutic Radiology and Oncology (ASTRO) Meeting in Boston.

The findings show there were additional benefits to acupuncture treatment for breast cancer patients, such as an increased sense of well being, more energy, and in some cases, a higher sex drive, that were not experienced in those patients who underwent drug treatment for their hot flashes.
The reduction in hot flashes lasted longer for those breast cancer patients after completing their acupuncture treatment, compared to patients after stopping their drug therapy plan.
Approximately eighty percent of women treated for breast cancer suffer from hot flashes after being treated with chemotherapy and/or anti-estrogen hormones, such as tamoxifen and Arimidex. Although HRT is typically used to relieve these symptoms, breast cancer patients cannot use this therapy because it may increase the risk of the cancer coming back.
Patients are often treated with steroids and/or antidepressant drugs instead. These drugs, however, have additional side effects, such as weight gain, nausea, constipation and fatigue. The antidepressant, venlafaxine (Effexor), a selective serotonin reuptake inhibitor, is one of the most common drugs used to treat hot flashes. However, many women decide against this treatment choice because of potential side effects, including decreased libido, insomnia, dizziness and nausea, or because they do not want to take any additional medications.
At ASTRO, the randomized clinical trial compared acupuncture treatment to venlafaxine for 12 weeks to find out if acupuncture reduced vasomotor symptoms in breast cancer patients receiving hormonal therapy and produced fewer side effects than venlafaxine. The study included 47 breast cancer patients who received either tamoxifen or Arimidex and had at least 14 hot flashes per week.
Overall, the results demonstrated that acupuncture reduces hot flashes as effectively as venlafaxine, with no side effects, and also provides additional health benefits to patients. This approach, although unusual, offers a safe and effective option for women with hormone-sensitive breast cancer.

The findings show there were additional benefits to acupuncture treatment for breast cancer patients, such as an increased sense of well being, more energy, and in some cases, a higher sex drive, that were not experienced in those patients who underwent drug treatment for their hot flashes.
The reduction in hot flashes lasted longer for those breast cancer patients after completing their acupuncture treatment, compared to patients after stopping their drug therapy plan.
Approximately eighty percent of women treated for breast cancer suffer from hot flashes after being treated with chemotherapy and/or anti-estrogen hormones, such as tamoxifen and Arimidex. Although HRT is typically used to relieve these symptoms, breast cancer patients cannot use this therapy because it may increase the risk of the cancer coming back.
Patients are often treated with steroids and/or antidepressant drugs instead. These drugs, however, have additional side effects, such as weight gain, nausea, constipation and fatigue. The antidepressant, venlafaxine (Effexor), a selective serotonin reuptake inhibitor, is one of the most common drugs used to treat hot flashes. However, many women decide against this treatment choice because of potential side effects, including decreased libido, insomnia, dizziness and nausea, or because they do not want to take any additional medications.
At ASTRO, the randomized clinical trial compared acupuncture treatment to venlafaxine for 12 weeks to find out if acupuncture reduced vasomotor symptoms in breast cancer patients receiving hormonal therapy and produced fewer side effects than venlafaxine. The study included 47 breast cancer patients who received either tamoxifen or Arimidex and had at least 14 hot flashes per week.
Overall, the results demonstrated that acupuncture reduces hot flashes as effectively as venlafaxine, with no side effects, and also provides additional health benefits to patients. This approach, although unusual, offers a safe and effective option for women with hormone-sensitive breast cancer.
Sunday, September 21, 2008
Can a vaccine help fight breast cancer?
A new vaccine in development targets breast cancers that proliferate aggressively in response to the growth factor HER-2. About 25-30% of women with breast cancer have HER-2 positive tumours. Herceptin (trastuzumab), a man-made antibody approved for the treatment of breast cancer, targets these cancers. However, tumour cells often become resistant to Herceptin over time. The experimental breast cancer vaccine makes mice reject tumours, even in cancers that are no longer sensitive to Herceptin.
The researchers published in the findings in Cancer Research and found that the vaccine elicits immune responses that kill HER-2 positive breast tumours in mice, irrespective of whether they become Herceptin resistant. If immune cells are properly primed by immunisation, then the cells can be destroyed.
The vaccine, developed at the Karmanos Cancer Center in Detroit, used DNA that carries the genetic code for a key piece of the HER-2 molecule. After injection of the DNA into the skin, a small electric pulse is administered to help cells take up the DNA and produce the protein that elicits immune responses.
Mice given the vaccine made anti-HER-2 antibodies. The vaccine also primed cellular immune responses that attacked breast cancer tumours. These cellular responses alone were enough to kill HER-2 positive cells in mice unable to make antibodies.
A version of the vaccine is now undergoing human safety tests.
A different HER-2 vaccine made headlines earlier this year when it halved the number of deaths in women with HER-2 positive breast cancer. The vaccine also slowed breast cancer recurrence. However, the researchers found that 26 months after vaccination, there was no significant difference in cancer recurrence between vaccinated and unvaccinated women.
In the long run, it is vitally important to test the animal research in humans to determine which approaches are valid and which are not. Early promising results do not always translate into survival advantages, but as we learn more about the science and biology of cancer, the technical approaches can only improve.
The concept behind using the bodies immune system to fight disease, including cancer, is a solid one but getting vaccines to work has proven elusive so far in solid tumours. More promising results have been seen in immune-related cancers such as NHL, but as the technology improves, we may one day see some advances in women with breast cancer.
The researchers published in the findings in Cancer Research and found that the vaccine elicits immune responses that kill HER-2 positive breast tumours in mice, irrespective of whether they become Herceptin resistant. If immune cells are properly primed by immunisation, then the cells can be destroyed.
The vaccine, developed at the Karmanos Cancer Center in Detroit, used DNA that carries the genetic code for a key piece of the HER-2 molecule. After injection of the DNA into the skin, a small electric pulse is administered to help cells take up the DNA and produce the protein that elicits immune responses.
Mice given the vaccine made anti-HER-2 antibodies. The vaccine also primed cellular immune responses that attacked breast cancer tumours. These cellular responses alone were enough to kill HER-2 positive cells in mice unable to make antibodies.
A version of the vaccine is now undergoing human safety tests.
A different HER-2 vaccine made headlines earlier this year when it halved the number of deaths in women with HER-2 positive breast cancer. The vaccine also slowed breast cancer recurrence. However, the researchers found that 26 months after vaccination, there was no significant difference in cancer recurrence between vaccinated and unvaccinated women.
In the long run, it is vitally important to test the animal research in humans to determine which approaches are valid and which are not. Early promising results do not always translate into survival advantages, but as we learn more about the science and biology of cancer, the technical approaches can only improve.
The concept behind using the bodies immune system to fight disease, including cancer, is a solid one but getting vaccines to work has proven elusive so far in solid tumours. More promising results have been seen in immune-related cancers such as NHL, but as the technology improves, we may one day see some advances in women with breast cancer.
Thursday, September 11, 2008
As a mark of respect
There will be no blog posts today in memory of those who died on 9/11 seven years ago.
Blogging will resume tomorrow.
Blogging will resume tomorrow.
Monday, September 8, 2008
Former NBA star Charles Barkley helps educate on colonoscopies
I was a bit surprised to read that the former 76'ers star, Charles Barkley, agreed to have a colonoscopy televised for Stand Up To Cancer last week, but it certainly generated some increased awareness and interest, even if he did become the butt of some obvious jokes.

You can see more about the newsworthy event HERE.

You can see more about the newsworthy event HERE.
Labels:
cancer,
colon cancer,
colonoscopy,
oncology,
pharma,
strategy
Wednesday, August 20, 2008
Is Gardasil cost effective?
Several strains of human papillomavirus (HPV) can cause cervical cancer, and two vaccines directed against the currently most important oncogenic strains (i.e., the HPV-16 and HPV-18 serotypes) have been developed.
Despite promising results from clinical trials, sufficient evidence of an effective long term vaccine against cervical cancer is lacking and the overall effect of the vaccines on cervical cancer remains unknown; the real impact of HPV vaccination on cervical cancer will not be known for decades.
The first vaccine against the HPV virus (Gardasil, Merck & Co) was licensed in 2006 for use in girls and women ages 9 to 26. Health officials recommend it for girls at age 11 or 12, and some doctors offer it to women in their 20s in "catch-up" vaccination campaigns. Merck also wants to market it to women ages 27 to 45, but so far the U.S. Food and Drug Administration has denied that request.
Gardasil is given in three doses over six months and costs about $375. It targets the two types of HPV, believed to be responsible for about 70 percent of cervical cancer cases, and two other types that cause most genital warts. The virus is spread by sexual activity.
Health officials say it's best to give the shots to girls at age 11 or 12, before they begin having sex. Some parents think that age is too young for a vaccination campaign against a sexually transmitted disease. But that is when the shots make the most economic sense, researchers found.
In the current edition of the New England Journal of Medicine, researchers used computer models to predict the health outcomes of girls and women who get the vaccination as well as Pap tests or other screenings, which are still recommended for vaccine recipients. Their calculation included the cost of the vaccine, screenings and treating cervical cancer and other illnesses targeted by the vaccine.
To determine cost-effectiveness, they used widely accepted economic measures of how much society is willing to pay to extend the life of a person by a year. They set a figure of $43,600 per year for the Gardasil vaccination of each 12-year-old girl, well below the $100,000 mark seen as an upper range for cost-effectiveness. However, the assumption is that the vaccine gives lifetime protection, which we don't know is true because the drug is too new and the data too preliminary.
The trends in the analysis suggested that as you get older, the vaccine becomes less cost-effective. This would imply that the earlier a female is vaccinated, the better the odds she will avoid HPV-caused cervical disease, thus lowering health-care costs in the long run.
References:
New England Journal of Medicine (free full text)
CNN
Despite promising results from clinical trials, sufficient evidence of an effective long term vaccine against cervical cancer is lacking and the overall effect of the vaccines on cervical cancer remains unknown; the real impact of HPV vaccination on cervical cancer will not be known for decades.
The first vaccine against the HPV virus (Gardasil, Merck & Co) was licensed in 2006 for use in girls and women ages 9 to 26. Health officials recommend it for girls at age 11 or 12, and some doctors offer it to women in their 20s in "catch-up" vaccination campaigns. Merck also wants to market it to women ages 27 to 45, but so far the U.S. Food and Drug Administration has denied that request.
Gardasil is given in three doses over six months and costs about $375. It targets the two types of HPV, believed to be responsible for about 70 percent of cervical cancer cases, and two other types that cause most genital warts. The virus is spread by sexual activity.
Health officials say it's best to give the shots to girls at age 11 or 12, before they begin having sex. Some parents think that age is too young for a vaccination campaign against a sexually transmitted disease. But that is when the shots make the most economic sense, researchers found.
In the current edition of the New England Journal of Medicine, researchers used computer models to predict the health outcomes of girls and women who get the vaccination as well as Pap tests or other screenings, which are still recommended for vaccine recipients. Their calculation included the cost of the vaccine, screenings and treating cervical cancer and other illnesses targeted by the vaccine.
To determine cost-effectiveness, they used widely accepted economic measures of how much society is willing to pay to extend the life of a person by a year. They set a figure of $43,600 per year for the Gardasil vaccination of each 12-year-old girl, well below the $100,000 mark seen as an upper range for cost-effectiveness. However, the assumption is that the vaccine gives lifetime protection, which we don't know is true because the drug is too new and the data too preliminary.
The trends in the analysis suggested that as you get older, the vaccine becomes less cost-effective. This would imply that the earlier a female is vaccinated, the better the odds she will avoid HPV-caused cervical disease, thus lowering health-care costs in the long run.
References:
New England Journal of Medicine (free full text)
CNN
Saturday, July 26, 2008
BRCA mutations in breast cancer
Genetic testing for BRCA1 and BRCA2 mutations can provide important information for women who are concerned about their breast and ovarian cancer risks and need to make relevant prevention and medical management decisions.
To date, lifetime risks of breast cancer in individual BRCA1/2 mutation carriers have been challenging to apply in clinical decision making. Published risk estimates vary significantly and are very dependent on the characteristics of the population under study. You can read more about in this article (free PDF download).
Another study interpreted validated functional data from the transactivation activity of BRCA1 in combination with analysis of protein modelling based on the structure of BRCA1 BRCT domains. With additional clinical and structural evidence, they were able to classify all missense variants in the BRCA1 COOH-terminal region. These results brought functional assays for BRCA1 closer to clinical applicability.
Cancer risks in a population-based study of BRCA1/2 mutation carriers have been recently estimated, but the numbers are still in their infancy and may be influenced by different risk factors. It is likely that there is broad variation in breast cancer risk among carriers of BRCA1 and BRCA2 mutations and ethnicity may also confer differences in risks associated with BRCA mutations.
To date, lifetime risks of breast cancer in individual BRCA1/2 mutation carriers have been challenging to apply in clinical decision making. Published risk estimates vary significantly and are very dependent on the characteristics of the population under study. You can read more about in this article (free PDF download).
Another study interpreted validated functional data from the transactivation activity of BRCA1 in combination with analysis of protein modelling based on the structure of BRCA1 BRCT domains. With additional clinical and structural evidence, they were able to classify all missense variants in the BRCA1 COOH-terminal region. These results brought functional assays for BRCA1 closer to clinical applicability.
Cancer risks in a population-based study of BRCA1/2 mutation carriers have been recently estimated, but the numbers are still in their infancy and may be influenced by different risk factors. It is likely that there is broad variation in breast cancer risk among carriers of BRCA1 and BRCA2 mutations and ethnicity may also confer differences in risks associated with BRCA mutations.
Labels:
BRCA,
breast cancer,
cancer,
market intelligence,
market research,
market trends,
mutation,
oncology
Sunday, July 20, 2008
How is cancer formed?
All cancers start with mutations in one single cell. The mutations are located in the cell's DNA and may be inherited, although less than 10% of all cancer mutations are inherited. Usually, the mutation arises as a result of environmental factors.
The DNA mutation may be a single nucleotide change, or a deletion or duplication of the DNA sequence. A change in the genetic sequence can then lead to the production of a mutant protein.
 Image via WikipediaIn rare cases such as chronic myeloid leukemia (CML) or gastrointestinal stromal tumours (GIST) one mutation is enough, but in most cancers, it is usually an accumulation of mutations that irreversibly transforms a normal cell into a cancerous one. As we age, we accumulate more and more mutations as we are exposed to environmental carcinogens and this explains why cancer incidence increases with age.
Image via WikipediaIn rare cases such as chronic myeloid leukemia (CML) or gastrointestinal stromal tumours (GIST) one mutation is enough, but in most cancers, it is usually an accumulation of mutations that irreversibly transforms a normal cell into a cancerous one. As we age, we accumulate more and more mutations as we are exposed to environmental carcinogens and this explains why cancer incidence increases with age.
These mutations can disrupt the cell’s life cycle of growth, proliferation, and death. This leads to the accumulation of more “rogue” cancer cells and the development of a tumour mass.
Normal cells have a natural lifespan and eventually die, a process known as apoptosis, or programmed cell death. They are replaced by new cells and so the process is repeated. Cancer cells do not respond to the signals that regulate cell growth and division. Thus these cells grow unchecked, producing more and more cancer cells.
A cell may die because it is damaged or old. Once a cell is signaled to die, the cell makes proteases and enzymes that degrade its components. The DNA in the nucleus is fragmented, the cell membrane shrinks, and, eventually, a neighboring cell engulfs the cellular remains.
To grow beyond a certain size, tumours must transport nutrients in and excrete wastes. The cancer cells that make up a tumour attract blood vessels to grow into the tumour mass, a process known as angiogenesis. The blood vessels then nourish the tumour just like any organ in the body; because the tumour is made of your own cells, the body does not recognise it as foreign, in the way it would a virus or bacteria.
The age of a cell and its ability to divide is related to structures or telomeres. The telomeres are specialised sequences at the ends of each chromosome and they prevent end-to-end fusion of chromosomes. These telomeres protect the ends of chromosomal DNA from accidents.
As normal cells go through cycles of growth and division, their telomeric DNA gets shorter and shorter and shorter and ultimately so short it can no longer protect the ends of chromosomal DNA. Eventually, the telomeres start fusing, chromosomes start fusing in those cells, and those cells die.
Cancer cells must avoid this problem because they want to grow indefinitely. Instead of dying, they turn on an enzyme called telomerase that is normally expressed only early in embryologic development and in a small number of so-called stem cells in the body.
The telomerase enzyme is able to extend the telomeres, making them longer and longer thereby enabling the cancer cell to go through many cycles of growth and division without worrying about the imminent collapse of its telomeres. The telomerase ensures the telomeres stay very long and essentially protects them from harm.
Most of the deaths from cancer (90%) are due to cancer cells spreading and establishing colonies in other parts of the body, a process known as metastasis. To do that, inactivation of a whole series of controls that normally confines a cell to the site and the tissue where it normally grows occurs, enabling the cancer cells to move to other sites in the body.
Another interesting thing about cancer cells is that they are often different in shape and size to normal cells, and they no longer respond to signals that control normal cellular functions. Our body's immune response is constantly searching for these emerging pre-cancers or pre-tumour cells. Successful cancers have to avoid detection long enough to grow into a tumour.
The body has two adaptive immune responses, enabling it to adapt to changes in cells in our body, whether they be by infection or other changes, such as cancer. One of these responses is making antibodies produced by B cells, which bind and direct the elimination of those cells. The other response is the T cell immune response where T cells actually kill cells that are changed in the body. The body is in constant surveillance of the cells in our body, so that emerging pre-cancers or pre-tumour cells could be eliminated by the immune response.
So how does cancer arise in the first place?
Well, a cell carries the entire set of genetic instructions, the genome, that makes an entire organism. The instructions are encoded in DNA as genes and packaged as chromosomes in the nucleus. DNA is not indestructable and is subject to damage and mutations. Crucial changes in the genome affect the chance and rate of the development of a cancer cell.
A defining characteristic of cancer cells is that those cells have changes in the nature of the genes that are compared to the normal cells. These changes can be either mutations, or they can be deletion of whole genes, or they can be the addition of extra copies of genes. This is called genomic instability.
The changes in our genes that accumulate in cancer cells can be acquired by a number of mechanisms. One is that during the process of copying the genetic information, mistakes can be made. After the genetic information is copied, it has to be segregated to the two daughter cells. During that segregation process, it is often that the numbers of genes get distributed unevenly to those daughter cells. Cancer cells also have an inability to repair alterations in the DNA.
Overall, you need to acquire multiple changes in the genes or multiple genes, to get cancer, perhaps 5-7 genes on average. Those changes accumulate over a period of time. Some of those changes accelerate the rate of accumulation. A person might have inherited one gene change, for example, and others develop as we age. Developing these mutational changes will weaken the DNA and increase the risk of cancer developing.
Sources (downloadable PDF):
Hallmark of Cancer
The DNA mutation may be a single nucleotide change, or a deletion or duplication of the DNA sequence. A change in the genetic sequence can then lead to the production of a mutant protein.
 Image via WikipediaIn rare cases such as chronic myeloid leukemia (CML) or gastrointestinal stromal tumours (GIST) one mutation is enough, but in most cancers, it is usually an accumulation of mutations that irreversibly transforms a normal cell into a cancerous one. As we age, we accumulate more and more mutations as we are exposed to environmental carcinogens and this explains why cancer incidence increases with age.
Image via WikipediaIn rare cases such as chronic myeloid leukemia (CML) or gastrointestinal stromal tumours (GIST) one mutation is enough, but in most cancers, it is usually an accumulation of mutations that irreversibly transforms a normal cell into a cancerous one. As we age, we accumulate more and more mutations as we are exposed to environmental carcinogens and this explains why cancer incidence increases with age.These mutations can disrupt the cell’s life cycle of growth, proliferation, and death. This leads to the accumulation of more “rogue” cancer cells and the development of a tumour mass.
Normal cells have a natural lifespan and eventually die, a process known as apoptosis, or programmed cell death. They are replaced by new cells and so the process is repeated. Cancer cells do not respond to the signals that regulate cell growth and division. Thus these cells grow unchecked, producing more and more cancer cells.
A cell may die because it is damaged or old. Once a cell is signaled to die, the cell makes proteases and enzymes that degrade its components. The DNA in the nucleus is fragmented, the cell membrane shrinks, and, eventually, a neighboring cell engulfs the cellular remains.
To grow beyond a certain size, tumours must transport nutrients in and excrete wastes. The cancer cells that make up a tumour attract blood vessels to grow into the tumour mass, a process known as angiogenesis. The blood vessels then nourish the tumour just like any organ in the body; because the tumour is made of your own cells, the body does not recognise it as foreign, in the way it would a virus or bacteria.
The age of a cell and its ability to divide is related to structures or telomeres. The telomeres are specialised sequences at the ends of each chromosome and they prevent end-to-end fusion of chromosomes. These telomeres protect the ends of chromosomal DNA from accidents.
As normal cells go through cycles of growth and division, their telomeric DNA gets shorter and shorter and shorter and ultimately so short it can no longer protect the ends of chromosomal DNA. Eventually, the telomeres start fusing, chromosomes start fusing in those cells, and those cells die.
Cancer cells must avoid this problem because they want to grow indefinitely. Instead of dying, they turn on an enzyme called telomerase that is normally expressed only early in embryologic development and in a small number of so-called stem cells in the body.
The telomerase enzyme is able to extend the telomeres, making them longer and longer thereby enabling the cancer cell to go through many cycles of growth and division without worrying about the imminent collapse of its telomeres. The telomerase ensures the telomeres stay very long and essentially protects them from harm.
Most of the deaths from cancer (90%) are due to cancer cells spreading and establishing colonies in other parts of the body, a process known as metastasis. To do that, inactivation of a whole series of controls that normally confines a cell to the site and the tissue where it normally grows occurs, enabling the cancer cells to move to other sites in the body.
Another interesting thing about cancer cells is that they are often different in shape and size to normal cells, and they no longer respond to signals that control normal cellular functions. Our body's immune response is constantly searching for these emerging pre-cancers or pre-tumour cells. Successful cancers have to avoid detection long enough to grow into a tumour.
The body has two adaptive immune responses, enabling it to adapt to changes in cells in our body, whether they be by infection or other changes, such as cancer. One of these responses is making antibodies produced by B cells, which bind and direct the elimination of those cells. The other response is the T cell immune response where T cells actually kill cells that are changed in the body. The body is in constant surveillance of the cells in our body, so that emerging pre-cancers or pre-tumour cells could be eliminated by the immune response.
So how does cancer arise in the first place?
Well, a cell carries the entire set of genetic instructions, the genome, that makes an entire organism. The instructions are encoded in DNA as genes and packaged as chromosomes in the nucleus. DNA is not indestructable and is subject to damage and mutations. Crucial changes in the genome affect the chance and rate of the development of a cancer cell.
A defining characteristic of cancer cells is that those cells have changes in the nature of the genes that are compared to the normal cells. These changes can be either mutations, or they can be deletion of whole genes, or they can be the addition of extra copies of genes. This is called genomic instability.
The changes in our genes that accumulate in cancer cells can be acquired by a number of mechanisms. One is that during the process of copying the genetic information, mistakes can be made. After the genetic information is copied, it has to be segregated to the two daughter cells. During that segregation process, it is often that the numbers of genes get distributed unevenly to those daughter cells. Cancer cells also have an inability to repair alterations in the DNA.
Overall, you need to acquire multiple changes in the genes or multiple genes, to get cancer, perhaps 5-7 genes on average. Those changes accumulate over a period of time. Some of those changes accelerate the rate of accumulation. A person might have inherited one gene change, for example, and others develop as we age. Developing these mutational changes will weaken the DNA and increase the risk of cancer developing.
Sources (downloadable PDF):
Hallmark of Cancer
Monday, July 14, 2008
Colorectal cancer screenings still low
Colorectal cancer screening tests have been proven to reduce colorectal cancer mortality, but a recent National Health study showed that only about half of U.S. men and women 50 and older receive the recommended tests. Image via Wikipedia: endocopic screening of colon cancer
Image via Wikipedia: endocopic screening of colon cancer
The Centers for Disease Control and Prevention conducted a National Health Interview Survey and found only 50 percent of men and women 50 and older had received screening in 2005. Although this was an improvement over the 43 percent of screened individuals reported in 2000, it is still suboptimal.
Colorectal cancer is one of the leading cancer killers in the United States, behind only lung cancer. Screening has been shown to significantly reduce mortality from colorectal cancer, but a lot of people are still not getting screened.
A major problem could be insurance coverage in the US. Among people without health insurance, the rate of colorectal cancer screening was 24.1 percent compared to over 50 percent of insured Americans, depending on the type of insurance. Among patients without a usual source of health care, the screening rate was 24.7 percent compared to 51.9 percent of patients with a usual source of health care.
The increase in colorectal cancer screening rates observed from 2000 to 2005 may have been due in part to increased media coverage of the importance of colonoscopy as a measure to prevent cancer and detect it early. Other factors for the increase include the fact that Medicare expanded its coverage for colonoscopy screenings to a wider range of patients in 2001.
 Image via Wikipedia: endocopic screening of colon cancer
Image via Wikipedia: endocopic screening of colon cancerThe Centers for Disease Control and Prevention conducted a National Health Interview Survey and found only 50 percent of men and women 50 and older had received screening in 2005. Although this was an improvement over the 43 percent of screened individuals reported in 2000, it is still suboptimal.
Colorectal cancer is one of the leading cancer killers in the United States, behind only lung cancer. Screening has been shown to significantly reduce mortality from colorectal cancer, but a lot of people are still not getting screened.
A major problem could be insurance coverage in the US. Among people without health insurance, the rate of colorectal cancer screening was 24.1 percent compared to over 50 percent of insured Americans, depending on the type of insurance. Among patients without a usual source of health care, the screening rate was 24.7 percent compared to 51.9 percent of patients with a usual source of health care.
The increase in colorectal cancer screening rates observed from 2000 to 2005 may have been due in part to increased media coverage of the importance of colonoscopy as a measure to prevent cancer and detect it early. Other factors for the increase include the fact that Medicare expanded its coverage for colonoscopy screenings to a wider range of patients in 2001.
Subscribe to:
Posts (Atom)
![Reblog this post [with Zemanta]](http://img.zemanta.com/reblog_e.png?x-id=a0e82ef2-62e8-44f1-9d90-1a3e4dc48e2f)
![Reblog this post [with Zemanta]](http://img.zemanta.com/reblog_e.png?x-id=d21de68e-1289-4c8e-83da-df0524b04d2f)
![Reblog this post [with Zemanta]](http://img.zemanta.com/reblog_e.png?x-id=b6cc0bfe-157f-4d66-a3ad-c2c843ce600d)




